LMU Newsroom
What is going on at LMU? Everything at a glance in the LMU Newsroom — news, events, interviews, backgrounds, stories.

A handle on the stuff that never gets read
Professor Julian Schröter, a new face at LMU, takes a digital approach to studying literature.
Read more
Activation of innate immunity: Important piece of the puzzle identified
LMU researchers have deciphered the complex interplay of various enzymes around an innate immune receptor, which plays an important role in defending our bodies against viruses.
Read more
Where budding media talent learns the ropes
Mediaschool Bayern is a place where students can experiment and hone their skills. Live and in front of an audience. Its flagship: the radio station M94.5.
Read moreINSIGHTS. Magazine

"Really?" - the new issue of INSIGHTS
"Echt jetzt" - the new issue of EINSICHTEN The new edition of the research magazine EINSICHTEN is out, with the main topic: "Really? Is the boundary between natural and artificial increasingly fading?" Click here for the highlights.
Read more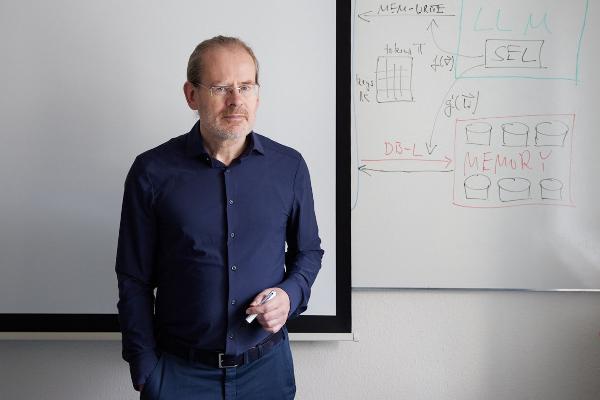
Polyglot machines
How artificial intelligence learns the rich variety of human languages: Hinrich Schütze, computational linguist at LMU, researches multilingual software that can do small languages. From the research magazine EINSICHTEN
Read more
From the steppe to the city
A social transformation is underway in Mongolia, as many nomadic pastoralists move to urban areas. Geographer Lukas Lehnert investigates what this means for the environment and ecosystems. From the research magazine EINSICHTEN
Read more